(Almost) Final Bill of Materials Published
Luther Johnson • November 21, 2020
From soup to nuts, everything in the box
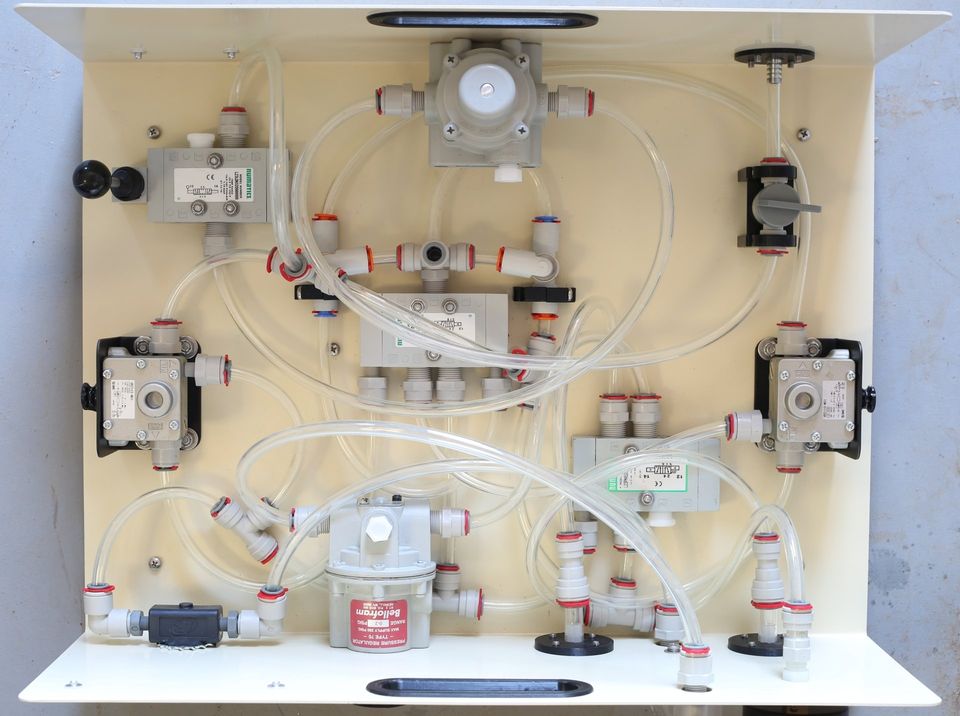
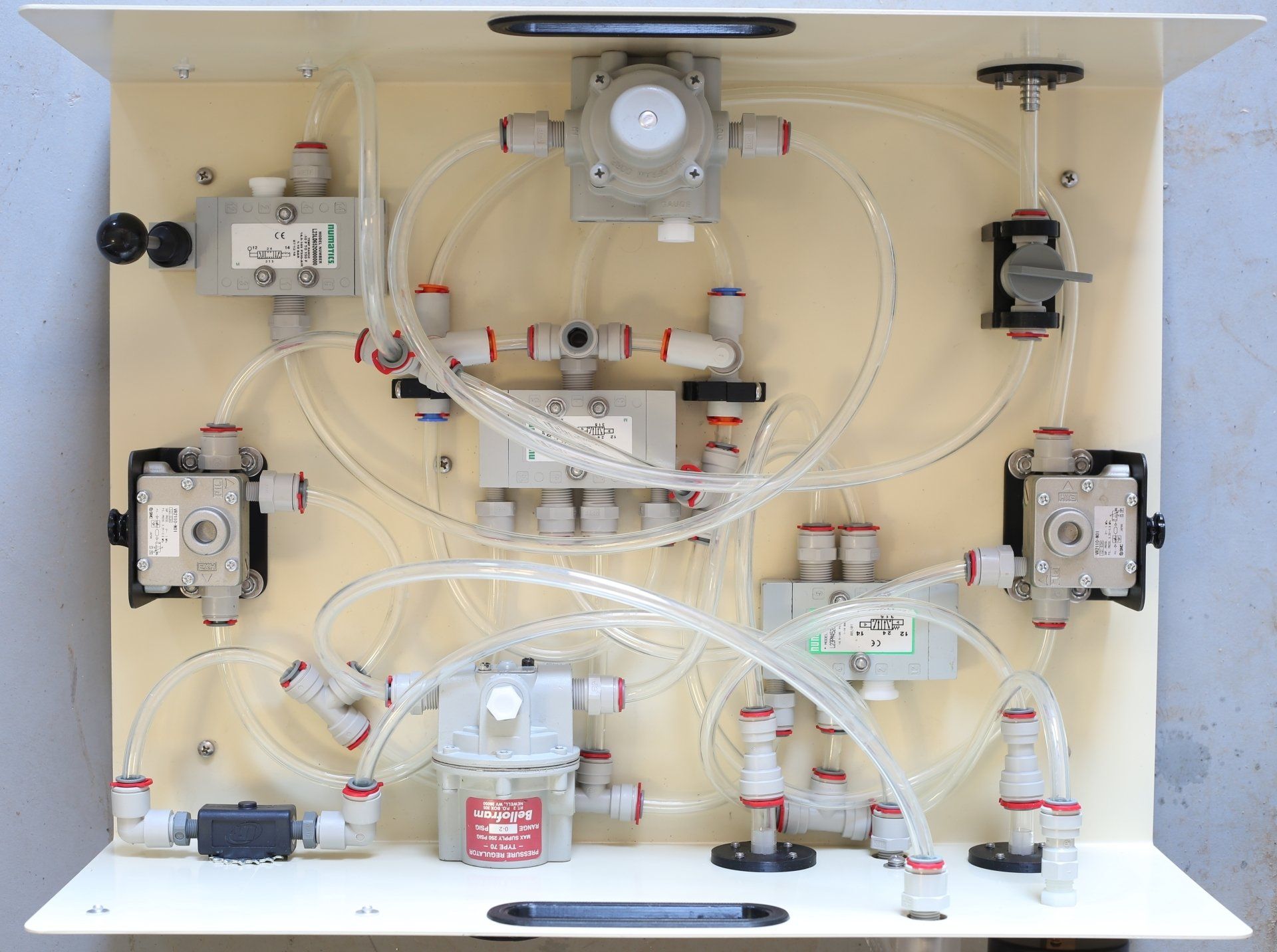
The bill of materials is finalized, there are no changes in the pneumatic components (there can't be, because we need to make the machine identical, in the materials that contact airflow, and in its operation, to the machine that was tested), but now we've put up all the other details, like the steel enclosure, the 3D-printed fittings, and all the various fasteners, so there is a complete public list of everything in the machine, and where to buy every single part.
Some of the manufacturers and manufacturer part numbers are not listed, because in those cases, that information is proprietary to the distributors we purchased from. We did receive that information for all of the pneumatic parts, because we needed it to do the bio-compatibility review, to research the manufacturer information on materials and certifications, but we agreed not to make that source information public.
(That is a distributor's business, after all, finding the things we need that we don't know how to find, and making it easy for us to buy them, in the quantities we need - and the people we've worked with have been wonderful. We would particularly like to thank McMaster-Carr, who have been extremely supportive of this project from the beginning.)
The one item that is listed, but which we haven't made yet, and therefore for which we don't yet have a picture, is an additional information placard that we need to attach, to comply with FDA regulations, for the device that will (in time) be approved for sale or distribution. This will go on the back of the machine, in the upper right-hand corner. It will have company information (approved machines will at first be made and distributed by Luther Johnson's company, MakerLisp), and any other warnings or usage information required - we're still sorting that out.
See the bill of materials here.
We didn't want to mention the organization that was doing this safety testing, until we had the official reports, signed off and delivered, ready for publication (we thought of saying it was an U nusually L arge testing laboratory, but decided to just wait). Well, we received the completed reports earlier this month, and so we've put them on the Technical page. You can see them here . The tests of particulate and volatile organic compound emissions, and analysis of the risk of health effects, were done by UL. UL is considered by many to be the world leader in safety science. We are confident that we have reliable results. The biocompatibility analysis was done, evaluating emissions from the ventilator, considering the case of 'permanent exposure'. The ventilator is specified for use with adults only, so all patients would be at least 18 years of age. Given an average lifespan of 70 years, that works out to 52 years on the ventilator, 24 hours a day. We're not allowed to publish excerpts, we can only make the reports available in full (however there is a summary on page 2 of the Biocompatibility Evaluation report). So of course there is more performance and endurance testing to do, and there are many other regulatory compliances to achieve, but we're in very good shape on the matter of meeting standards for safety of breathable gas.

We've made a WHOLE BUNCH of new videos showing how the MakerVent Model T can be connected to many commonly available, generic, breathing circuit components, to effect a variety of ventilation modes. Many of these components are parts of common BVM (bag-valve-mask) resuscitators, but they're all easy to get and there are many functionally equivalent alternatives, availlable from many sources. A key feature that makes this possible is the way the spring ("patient safety") valve works, and its connection to the auxiliary port. Watch here .

We reached our goal early, and now we're busily working on getting the process and paperwork ready so that we can build ventilators in an approved manner - documenting it all as part of the request for authorization. We're working with regulatory consultants, and our teammates at U.S. Clean Lab to get all of the details of producing machines in a repeatable, accountable, and traceable way. Thanks again to all of our lenders !

We are currently running a crowdfunding campaign on Kiva, a "micro-finance" platform, in order to support our effort to achieve FDA authorization - this is not a donation, it's a loan - you can lend as little as $25, and you get paid back, a little every month. Please check out our campaign . Thanks !

Lee Collier, an intensive care doctor in London, UK, has successfully built his MakerVent. Lee is using his machine to gather pressure, flow, and other data, to characterize the behavior of the MakerVent under various uses and conditions. Read more about Lee on the Team page. Here's a video of Lee's machine in action !

The updated cleaning process was completed on 7/2/2020, and I shipped the machine to Marietta, Georgia yesterday, 7/3/2020. The machine will arrive in a week, and testing will take some weeks, but when it is done, we'll have an assessment of the safety of breathable gas for a patient under a prolonged exposure. If the tests fail, we'll get indications of what's happening, and of course we'll make adjustments in components and/or cleaning processes in order to pass the next round of tests, but we're hoping that we made good choices already, and that the machine will pass. In the meantime, I can now (nearly) fully focus on the FDA EUA (Emergency Use Authorization). This requires quite a bit of documentation and institution of production and quality processes to ensure that all the machines produced according to this design and set of specifications, safely perform according to the indicated standards. I'm getting some consulting help here, but it is still a big effort.

We discovered some changes to the cleaning protocol required - one of the detergents used to clean the regulators was a little too strong, and was taking off some of the anti-corrosion, anti-oxidation coating on the cast aluminum bodies of the regulators - we've researched this coating, and are satisfied that is bio-compatible for breathable air, but we don't want any excess particles getting into the airflow, so we're adjusting the cleaning solutions and procedure. If we don't do this well, it will fail when we send the machine to the testing lab for ISO 18562 compliance - it's good to know that it would have been caught then, but of course it's better, faster, and cheaper, to catch it and make the adjustments now. There is now a tubing connection list on the Technical page, which explains how to hook everything up, and there are new videos that explain the terms used in that list. In those videos, I said there were 31 connections total, 29 involving the 3/8" OD, 1/4" ID tubing. Well, yes, but two of those connections, the center ports on tees 1 and 2, are alternative endpoints for the tube coming out of the lever valve (machine operating on/off) output, and don't really represent a separate tube, so now the list just describes the 27 3/8" OD, 1/4" ID tubes, and the two 1/2" OD, 3/8" ID tubes. The lengths of the tubes chosen are for the the layout of the components in the enclosure we have made, to date. I had thought, at the time of making the video, that there would be two lists describing the tubing, but there is just one, with all the necessary information required, to hook up the machine as depicted and demonstrated here.
The ventilator is at an oxygen-cleaning service in Tucson, it's expected that this cleaning will be done on 6/12/2020. After that, the machine goes to a testing lab to evaluate the safety of breathable gas passing through it, according to ISO 18562. The first ventilator unit's enclosure and related component mounting hardware was hand-crafted, as we experimented with and adjusted the configuration of the machine. Now, production of a second unit is under way, at a local metal shop, built directly from the latest CAD data that reflects all the most recent physical adjustments we have made. We will soon have assembly instructions, CAD data, and a full bill of materials for the enclosure and mounting hardware posted, accessible from the technical page. The next few complete units that we produce, will be built from component parts and enclosures we purchase and ship to the cleaning lab, which will then be cleaned and assembled into the finished ventilator, onsite, at that lab. As we flesh out the details of this process, we'll make the information available here, for any builder to contract with that lab to get parts cleaned and/or assembled in the same way. We will also publish all the cleaning protocol documentation, so that other services can clean component parts to the same standards.